Most Important Questions of All The Chaptar of Class 12th Below are the important questions along with the answers .These questions are most asked in the recent school exams. These are totally based on the CBSE board curriculum. We recommend everyone, do not skip these questions.
Important Questions of All The Chaptar of Class 12th
Solution
1 Which of the following solutions will have the highest conductivity at
(a)
(b)
(c)
(d)
2 A
3 Answer the following questions:
a. State Henry's law and explain why are the tanks used by scuba divers filled with air diluted with helium (
b. Assume that argon exerts a partial pressure of 6 bar. Calculate the solubility of argon gas in water.
(Given Henry's law constant for argon dissolved in water,
Electrochemistry
- Given below are two statements labelled as Assertion (A) and Reason (R)
Assertion (A): During electrolysis of aqueous copper sulphate solution using copper electrodes hydrogen gas is released at the cathode.
Reason (R): The electrode potential ofCu^(2+)//Cu \mathrm{Cu}^{2+} / \mathrm{Cu} H+//H_(2) \mathrm{H}+/ \mathrm{H}_2
Select the most appropriate answer from the options given below:
(a) BothA A R R R R A A
(b) BothA A R R R R A A
(c)A A R R
(d)A A R R - (a) Can we construct an electrochemical cell with two half-cells composed of
ZnSO_(4) \mathrm{ZnSO}_4
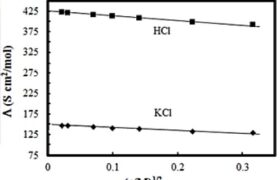
(b) Calculate thelambda^(0)m \lambda^0 \mathrm{~m} Cl \mathrm{Cl}
(c) The cell constant of a conductivity cell is 0.146cm^(-1) 0.146 \mathrm{~cm}^{-1} 0.01M 0.01 \mathrm{M} 298K 298 \mathrm{~K} 1000ohm 1000 \mathrm{ohm}
3. The molar conductivity ofCH_(3)COOH \mathrm{CH}_3 \mathrm{COOH} 390Scm^(2)//mol 390 \mathrm{Scm}^2 / \mathrm{mol} CH_(3)COOK \mathrm{CH}_3 \mathrm{COOK}
a.100Scm^(2)//mol 100 \mathrm{Scm}^2 / \mathrm{mol}
b.115Scm^(2)//mol 115 \mathrm{Scm}^2 / \mathrm{mol}
c.150Scm^(2)//mol 150 \mathrm{Scm}^2 / \mathrm{mol}
d.125Scm^(2)//mol 125 \mathrm{Scm}^2 / \mathrm{mol}
3. The molar conductivity of
a.
b.
c.
d.
Chemical Kinetics
1 Which of the following statement is true?
(a) molecularity of reaction can be zero or a fraction.
(b) molecularity has no meaning for complex reactions.
(c) molecularity of a reaction is an experimental quantity
(d) reactions with the molecularity three are very rare but are fast.
2 If the initial concentration of substanceA A 1.5M 1.5 \mathrm{M} A A 0.75M 0.75 \mathrm{M}
(a)0.00625molL^(-1)s^(-1) 0.00625 \mathrm{molL}^{-1} \mathrm{~s}^{-1}
(b)0.00625s^(-1) 0.00625 \mathrm{~s}^{-1}
(c)0.00578molL^(-1)s^(-1) 0.00578 \mathrm{molL}^{-1} \mathrm{~s}^{-1}
(d)0.00578s^(-1) 0.00578 \mathrm{~s}^{-1}
3 a. Radioactive decay follows first - order kinetics. The initial amount of two radioactive elementsX X Y Y 1gm 1 \mathrm{gm} X X Y Y
b. The hypothetical reactionP+Q rarr R P+Q \rightarrow R P P Q Q
(a) molecularity of reaction can be zero or a fraction.
(b) molecularity has no meaning for complex reactions.
(c) molecularity of a reaction is an experimental quantity
(d) reactions with the molecularity three are very rare but are fast.
2 If the initial concentration of substance
(a)
(b)
(c)
(d)
3 a. Radioactive decay follows first - order kinetics. The initial amount of two radioactive elements
b. The hypothetical reaction
D and F block
1 Match the properties with the elements of
(i) lowest enthalpy of atomization
(p) Sc
(ii) shows maximum number of oxidation states
(q)
(iii) transition metal that does not form coloured compounds
(r)
(s)
(a) (i) (r), (ii) (q), (iii) (p)
(b) (i) (r), (ii) (s), (iii) (p)
(c) (i) (p), (ii) (q), (iii) (r)
(d) (i) (s), (ii) (r), (iii) (p)
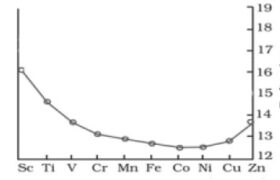
2 The trend of which property is represented by the following graph?
(a) ionization enthalpy
(b) atomic radii
(c) enthalpy of atomization
(d) melting point
3 Which of the following is not considered a transition element?
(a) Scandium
(b) Silver
(c) Vanadium
(d) Zinc
Coordination Compounds
1 (a) Write the formula for the following coordination compound
Bis(ethane-1,2-diamine) dihydroxidochromium(III) chloride
(b) Does ionization isomer for the following compound exist? Justify your answer.Hg[Co(SCN)_(4)] \mathrm{Hg}\left[\mathrm{Co}(\mathrm{SCN})_4\right]
(c) Is the central metal atom in coordination complexes a Lewis acid or a Lewis base? Explain.
2 The CFSE of[CoCl_(6)]^(3-) \left[\mathrm{CoCl}_6\right]^{3-} 18000cm^(-1) 18000 \mathrm{~cm}^{-1} [CoCl_(4)]^(-) \left[\mathrm{CoCl}_4\right]^{-}
a.18000cm^(-1) 18000 \mathrm{~cm}^{-1}
b.8000cm^(-1) 8000 \mathrm{~cm}^{-1}
c.2000cm^(-1) 2000 \mathrm{~cm}^{-1}
d.16000cm^(-1) 16000 \mathrm{~cm}^{-1}
3 The number of ions formed on dissolving one molecule ofFeSO_(4)*(NH_(4))_(2)SO_(4)*6H_(2)C \mathrm{FeSO}_4 \cdot\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4 \cdot 6 \mathrm{H}_2 \mathrm{C}
a. 3
b. 4
c. 5
d. 6
Bis(ethane-1,2-diamine) dihydroxidochromium(III) chloride
(b) Does ionization isomer for the following compound exist? Justify your answer.
(c) Is the central metal atom in coordination complexes a Lewis acid or a Lewis base? Explain.
2 The CFSE of
a.
b.
c.
d.
3 The number of ions formed on dissolving one molecule of
a. 3
b. 4
c. 5
d. 6
Haloalkanes and Haloarenes
1 (a) Arrange the isomeric dichlorobenzene in the increasing order of their boiling point and melting points.
(b) Explain why the electrophilic substitution reactions in haloarenes occur slowly and require more drastic conditions as compared to those in benzene.
2 Which one of the following compounds is more reactive towardsS_(N)1 \mathrm{S}_{\mathrm{N}} 1
a.CH_(2)=CHCH_(2)Br \mathrm{CH}_2=\mathrm{CHCH}_2 \mathrm{Br}
b.C_(6)H_(5)CH_(2)Br \mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{Br}
c.C_(6)H_(5)CH(C_(6)H_(5))Br \mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}\left(\mathrm{C}_6 \mathrm{H}_5\right) \mathrm{Br}
d.C_(6)H_(5)CH(CH_(3))Br \mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}\left(\mathrm{CH}_3\right) \mathrm{Br}
3 What would be the major product of the following reaction?
C_(6)H_(5)-CH_(2)-OC_(6)H5+HBrrarrA+B \mathrm{C}_6 \mathrm{H}_5-\mathrm{CH}_2-\mathrm{OC}_6 \mathrm{H} 5+\mathrm{HBr} \rightarrow \mathrm{A}+\mathrm{B}
a.A=C_(6)H_(5)CH_(2)OH,B=C_(6)H_(6) \mathrm{A}=\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{OH}, \mathrm{B}=\mathrm{C}_6 \mathrm{H}_6
b.A=C_(6)H_(5)CH_(2)OH,B=C6H5Br \mathrm{A}=\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{OH}, \mathrm{B}=\mathrm{C} 6 \mathrm{H} 5 \mathrm{Br}
c.A=C_(6)H_(5)CH_(3),B=C_(6)H_(5)Br \mathrm{A}=\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_3, \mathrm{~B}=\mathrm{C}_6 \mathrm{H}_5 \mathrm{Br}
d.A=C_(6)H_(5)CH_(2)Br,B=C_(6)H_(5)OH A=C_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{Br}, \mathrm{B}=\mathrm{C}_6 \mathrm{H}_5 \mathrm{OH}
(b) Explain why the electrophilic substitution reactions in haloarenes occur slowly and require more drastic conditions as compared to those in benzene.
2 Which one of the following compounds is more reactive towards
a.
b.
c.
d.
3 What would be the major product of the following reaction?
a.
b.
c.
d.
Download PDF File for more Important Questions with Answers – Click here
